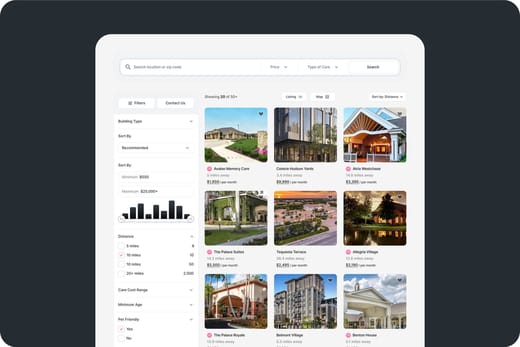
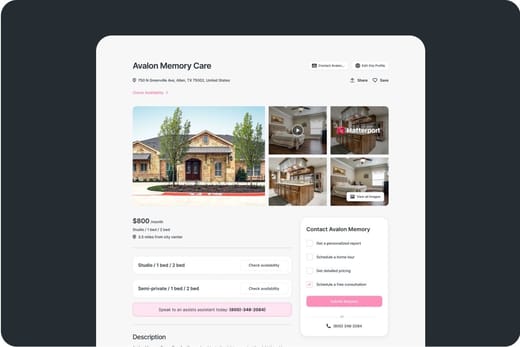
Inspection Reports for Corso Atlanta
3200 Howell Mill Rd, Atlanta, GA 30327, United States, GA, 30327
Back to Facility Profile
Inspection Report
Complaint Investigation
Deficiencies: 0
Sep 12, 2025
Visit Reason
The purpose of this visit was to investigate intake #GA50005054 with an onsite visit made on 2025-09-11 and inspection completed on 2025-09-12.
Findings
No deficiencies were cited as a result of this inspection.
Complaint Details
Investigation of intake #GA50005054; no deficiencies found.
Inspection Report
Complaint Investigation
Deficiencies: 0
Jul 21, 2025
Visit Reason
The purpose of this visit was to investigate intake #GA50003771 and #GA50004401.
Findings
No rule violations were cited as a result of this investigation.
Complaint Details
Investigation of intake #GA50003771 and #GA50004401 with no rule violations found.
Inspection Report
Complaint Investigation
Deficiencies: 0
Apr 27, 2025
Visit Reason
The purpose of this visit was to investigate intake GA 50002400.
Findings
No rules were cited as a result of this investigation which began on 2025-04-17 and ended on 2025-04-23.
Complaint Details
Investigation of intake GA 50002400; no deficiencies or citations were found.
Inspection Report
Complaint Investigation
Deficiencies: 0
Mar 31, 2025
Visit Reason
An onsite visit was made to this facility to investigate intakes GA 50001084 and GA 50001390 and conduct a compliance inspection.
Findings
No rules violations were cited as a result of this inspection and investigation.
Complaint Details
Investigation of intakes GA 50001084 and GA 50001390; no violations found.
Inspection Report
Complaint Investigation
Deficiencies: 0
Oct 11, 2024
Visit Reason
The purpose of this visit was to investigate intake #GA00250123.
Findings
No rule violations were cited as a result of this investigation.
Complaint Details
Investigation of intake #GA00250123 with no rule violations cited.
Inspection Report
Complaint Investigation
Deficiencies: 2
Aug 7, 2024
Visit Reason
The purpose of this visit was to investigate complaint intakes #GA00248586 and #GA00248705 through an unannounced inspection conducted on 8/7/2024.
Findings
The facility failed to return medication to proper secured storage following administration for one of three sampled residents and failed to provide medication administration services in accordance with physician orders for the same resident. Attempts to contact the responsible party were unsuccessful.
Complaint Details
Investigation of complaint intakes #GA00248586 and #GA00248705. The complaint was substantiated based on observations, record reviews, and interviews indicating medication administration and storage deficiencies.
Severity Breakdown
Level D: 2
Deficiencies (2)
| Description | Severity |
|---|---|
| Failed to return medication to proper secured storage following administration for Resident #1. | Level D |
| Failed to provide medication administration services in accordance with physician orders for Resident #1. | Level D |
Report Facts
Medication order date: Jun 16, 2024
Medication order quantity: 10
Medication administered: 1
Medication pills left: 3
Staff hire date: Feb 13, 2024
Attempts to contact: 3
Employees Mentioned
| Name | Title | Context |
|---|---|---|
| Staff C | Observed administering medication and interviewed regarding medication administration and storage deficiencies |
Inspection Report
Complaint Investigation
Deficiencies: 0
Dec 25, 2023
Visit Reason
The purpose of this visit was to investigate complaint intake # GA00241725.
Findings
There were no rule violations cited as a result of this survey.
Complaint Details
Complaint intake # GA00241725 was investigated with no rule violations cited.
Inspection Report
Complaint Investigation
Deficiencies: 0
Oct 20, 2023
Visit Reason
The purpose of this visit was to investigate intake #GA00239416 with an onsite visit made on 10/19/23 and inspection completed on 10/20/23.
Findings
No rule violations were cited during the inspection.
Complaint Details
Investigation of intake #GA00239416; no violations found.
Inspection Report
Complaint Investigation
Deficiencies: 0
May 18, 2023
Visit Reason
The purpose of this visit was to investigate intake #GA00234882 with an onsite visit conducted on 5/18/23.
Findings
The inspection was completed with no rule violations cited as a result of this investigation.
Complaint Details
Investigation of intake #GA00234882 resulted in no rule violations cited.
Inspection Report
Original Licensing
Deficiencies: 0
Jan 5, 2023
Visit Reason
The purpose of this visit was to conduct the initial inspection and to investigate intake #GA00229350.
Findings
No rule violations were cited as a result of this investigation.
Inspection Report
Complaint Investigation
Deficiencies: 0
Mar 15, 2022
Visit Reason
The purpose of this visit was to investigate intake GA00221845.
Findings
No rule violations were cited as a result of this investigation.
Complaint Details
Investigation of intake GA00221845 found no rule violations.
Inspection Report
Original Licensing
Deficiencies: 0
Dec 14, 2021
Visit Reason
The purpose of this visit was to conduct the initial inspection of the facility, which started on 2021-12-10 and was completed on 2021-12-14.
Findings
No rule violations were cited as a result of this inspection.
Loading inspection reports...